Sampling the conformational space of modified macrocyclic peptides by eNOES: models for cell permeability
Together with the group of Prof. Künzler and Prof. Güntert we propose a model for cell-permeation of modified macrocyclic peptides. The model is based on NMR structures which were obtained using a novel eNOE approach in various solvent mixtures. The solvent mixtures mimic various cellular compartments.
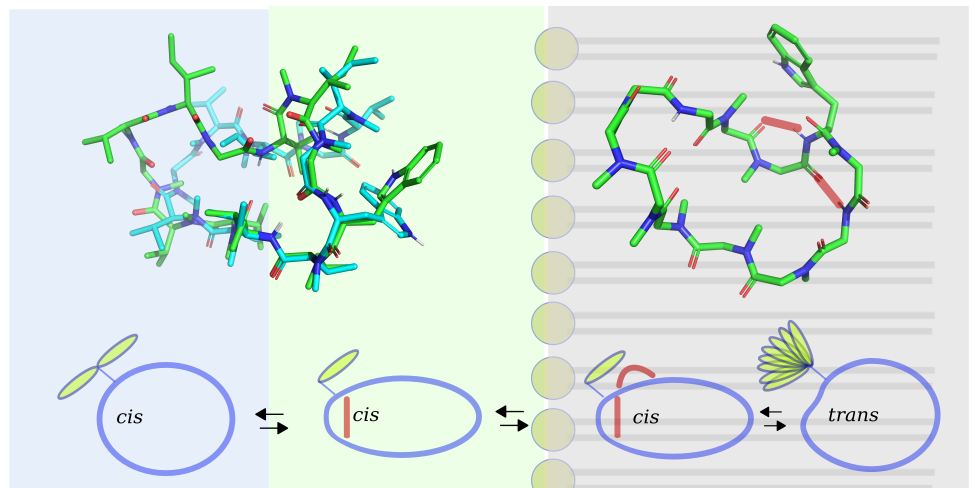
The biological activities and pharmacological properties of peptides and peptide mimetics are determined by their conformational states. Therefore, a detailed understanding of the conformational landscape is crucial for rational drug design. Nuclear magnetic resonance (NMR) is the only method for structure determination in solution. However, it remains challenging to determine the structures of peptides using NMR because of very weak nuclear Overhauser effects (NOEs), the semiquantitative nature of the rotating frame Overhauser effect (ROE), and the low number of NOEs/ROEs in N-methylated peptides. In this study, we introduce a new approach to investigating the structures of modified macrocyclic peptides. We utilize exact NOEs (eNOEs) in viscous solvent mixtures to replicate various cellular environments. eNOEs provide detailed structural information for highly dynamic modified peptides. Structures of high precision were obtained for cyclosporin A, with a backbone atom rmsd of 0.10 Å. Distinct conformational states in different environments were identified for omphalotin A (OmphA), a fungal nematotoxic and multiple backbone N-methylated macrocyclic peptides. A model for cell-permeation is presented for OmphA, based on its structures in polar, apolar, and mixed polarity solvents. During the transition from a polar to an apolar environment, OmphA undergoes a rearrangement of its H-bonding network, accompanied by a cis to trans isomerization of the ω torsion angle within a type VIa β-turn. We hypothesize that the kinetics of these conformational transitions play a crucial role in determining the membrane-permeation capabilities of OmphA.
The external pagepublications appeared in the Journal of the American Chemical Society.
